SENISCA is an award-winning biotech from the University of Exeter, focused on modulating RNA biology to treat age-related disease.
SENISCA targets the process of cellular ageing (senescence) via the development of RNA senotherapeutics which specifically target the causative molecular mechanisms that drive senescence.
Age-related disease is caused by the failure in basic cell health mechanisms, collectively known as the hallmarks of ageing. 15+ years of world-leading research by SENISCA's founders led to the identification of a novel hallmark of ageing, dysregulated RNA splicing, a fundamental contributor to cellular senescence and ageing. We are leveraging this proprietary knowledge to develop senotherapeutics that specifically reverse senescence for the treatment of age-related disease.






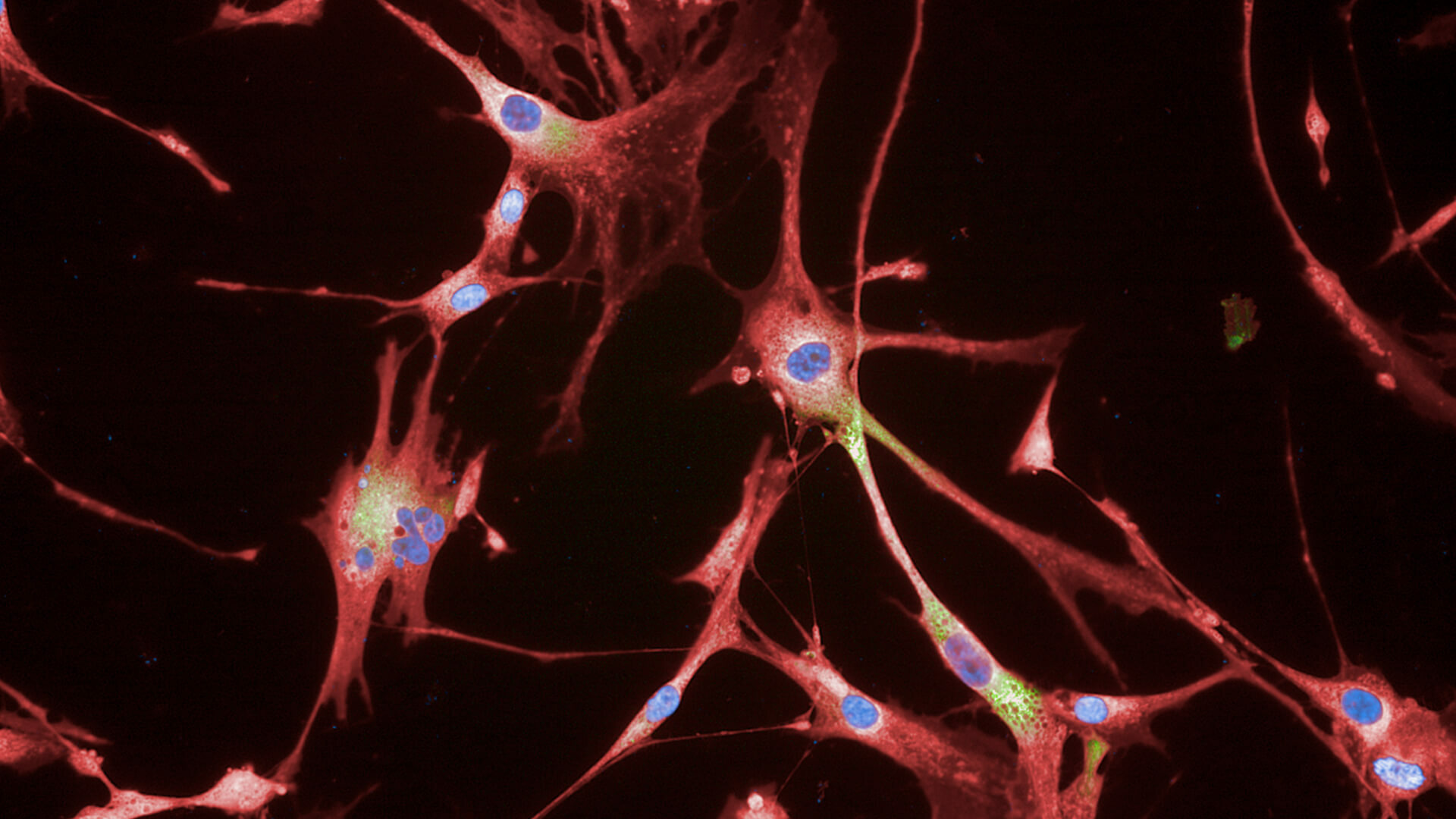 Rejuvenated Senescent Fibroblasts.
Rejuvenated Senescent Fibroblasts.










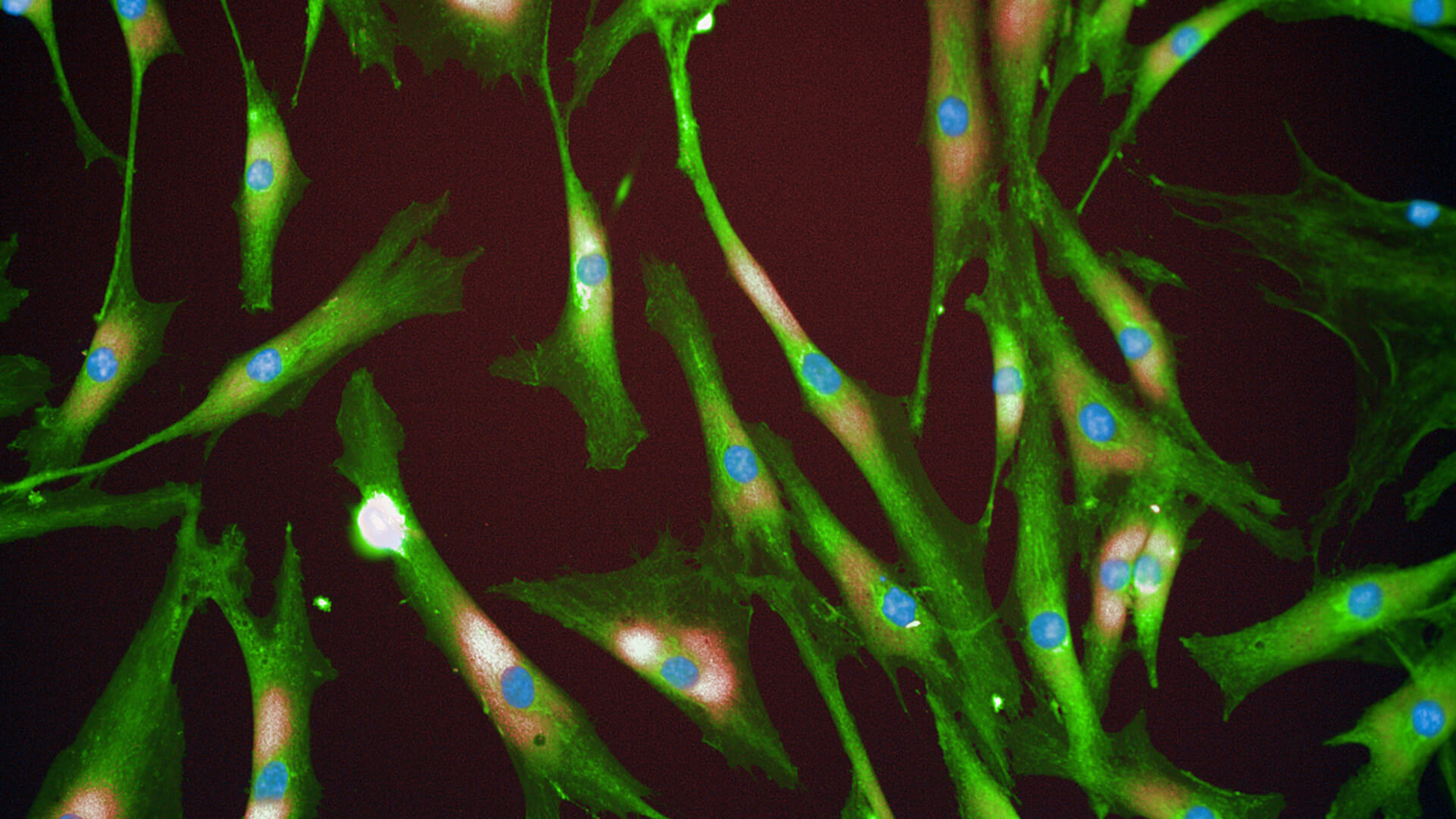 Senescent Fibroblast Cells.
Senescent Fibroblast Cells.
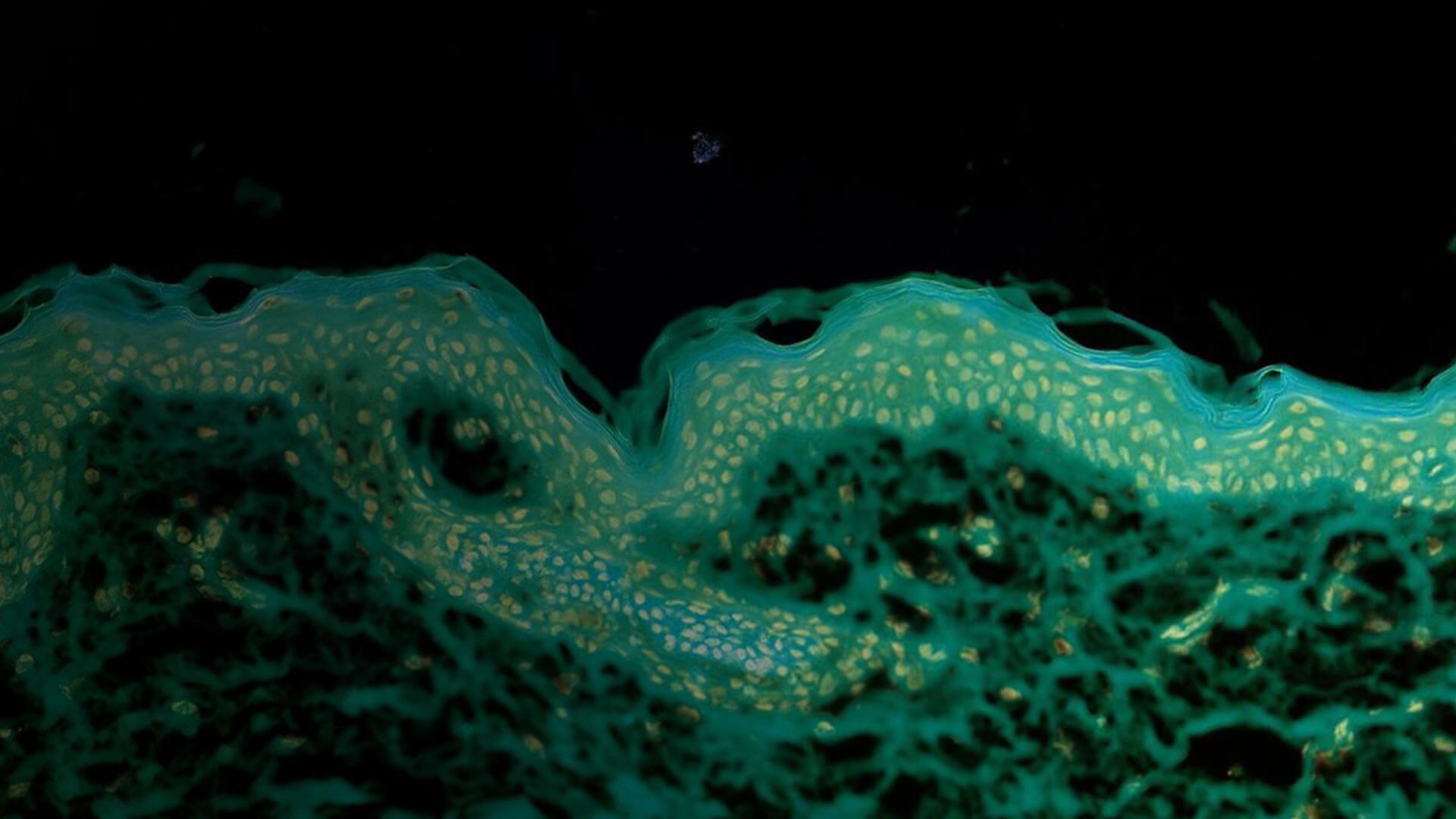 Stained human tissue section
Stained human tissue section